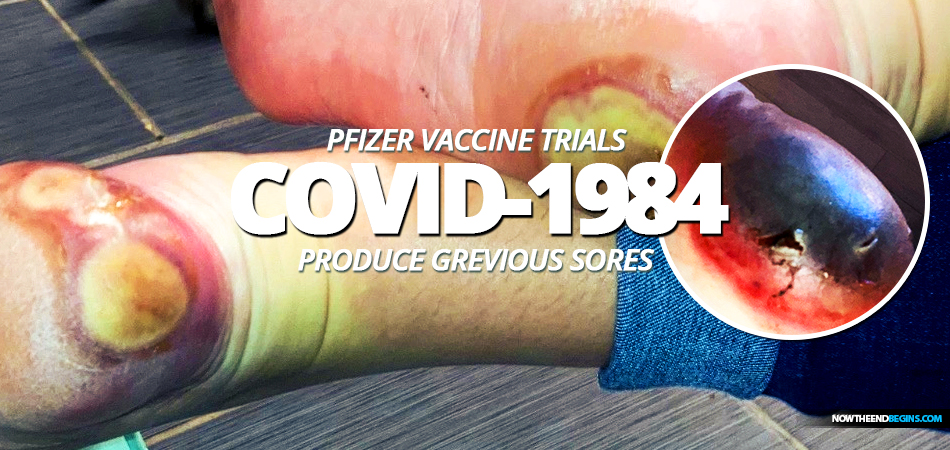
The injection reaction is called a "fixed drug eruption" and it is present on both feet. Patricia has been unable to walk without a cane since her emergency room visit on November 2nd.
A woman by the name of Patricia Chandler in Austin, Texas, volunteered to receive the new COVID-1984 vaccine from the joint partnership between Pfizer and BioNTech in Mainz, Germany. A giddy and gushing press release dated November 9th, 2020 says that the "vaccine candidate was found to be more than 90% effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysis" That all sounds really great until you take a look at the soles of Patricia Chandler's feet where you'll find oozing, crusting holes that look a whole lot like the 'grievous sores' described in Revelation.
"And I heard a great voice out of the temple saying to the seven angels, Go your ways, and pour out the vials of the wrath of God upon the earth. And the first went, and poured out his vial upon the earth; and there fell a noisome and grievous sore upon the men which had the mark of the beast, and upon them which worshipped his image." Revelation 16:1,2 (KJB)
Rightly dividing shows us that by the time you get to Revelation 16, the born again Church has already been raptured up-n-out and safely in Heaven for 12 chapters, so we know that these sores on this woman's feet cannot be caused by that. But man, what a great and horrific illustration of what's to come during the time of great Tribulation.
The GoFundMe page set up for her by her cousin tells us that the grievous sores reaction was incurred in a clinical trial for Pfizer/BioNTech's COVID-19 vaccine. The injection reaction is called a "fixed drug eruption" and it is present on both feet. She goes on to say that Patricia has been unable to walk without a cane since her emergency room visit on November 2nd. Are you starting to see why all the Big Pharma companies are immune from prosecution and have a taxpayer funded, billion dollar legal defense fund at their disposal?
This COVID-1984 vaccine may not be the Mark of the Beast, but I'll tell you what, I want nothing to do with it regardless of which pharmaceutical company it comes from. We did our best to verify the accuracy of this story before going to press, and have personally contributed to Patricia Chandler's GoFundMe. However, we advise you to do your due diligence and research it on your own before donating, should you feel so moved to do so. We hope to have either Patricia Chandler or her cousin Rebecca Moore who set up the fundraiser on our Prophecy Podcast program in the very near future, stay tuned for updates.

WARNING: I CAN NO LONGER SAY THAT BILL GATES PLANNED GLOBAL VACCINATIONS AND THE ID2020 DIGITAL IDENTIFICATION ARE NOT THE MARK OF THE BEAST
PFIZER AND BIONTECH ANNOUNCE VACCINE CANDIDATE AGAINST COVID-19 ACHIEVED SUCCESS IN FIRST INTERIM ANALYSIS FROM PHASE 3 STUDY
NEW YORK & MAINZ, GERMANY--(BUSINESS WIRE)-- Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced their mRNA-based vaccine candidate, BNT162b2, against SARS-CoV-2 has demonstrated evidence of efficacy against COVID-19 in participants without prior evidence of SARS-CoV-2 infection, based on the first interim efficacy analysis conducted on November 8, 2020 by an external, independent Data Monitoring Committee (DMC) from the Phase 3 clinical study.
After discussion with the FDA, the companies recently elected to drop the 32-case interim analysis and conduct the first interim analysis at a minimum of 62 cases. Upon the conclusion of those discussions, the evaluable case count reached 94 and the DMC performed its first analysis on all cases. The case split between vaccinated individuals and those who received the placebo indicates a vaccine efficacy rate above 90%, at 7 days after the second dose. This means that protection is achieved 28 days after the initiation of the vaccination, which consists of a 2-dose schedule. As the study continues, the final vaccine efficacy percentage may vary. The DMC has not reported any serious safety concerns and recommends that the study continue to collect additional safety and efficacy data as planned. The data will be discussed with regulatory authorities worldwide.
“Today is a great day for science and humanity. The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent COVID-19,” said Dr. Albert Bourla, Pfizer Chairman and CEO. “We are reaching this critical milestone in our vaccine development program at a time when the world needs it most with infection rates setting new records, hospitals nearing over-capacity and economies struggling to reopen. With today’s news, we are a significant step closer to providing people around the world with a much-needed breakthrough to help bring an end to this global health crisis. We look forward to sharing additional efficacy and safety data generated from thousands of participants in the coming weeks.”After discussion with the FDA, the companies recently elected to drop the 32-case interim analysis and conduct the first interim analysis at a minimum of 62 cases. Upon the conclusion of those discussions, the evaluable case count reached 94 and the DMC performed its first analysis on all cases. The case split between vaccinated individuals and those who received the placebo indicates a vaccine efficacy rate above 90%, at 7 days after the second dose. This means that protection is achieved 28 days after the initiation of the vaccination, which consists of a 2-dose schedule. As the study continues, the final vaccine efficacy percentage may vary. The DMC has not reported any serious safety concerns and recommends that the study continue to collect additional safety and efficacy data as planned. The data will be discussed with regulatory authorities worldwide.
“I want to thank the thousands of people who volunteered to participate in the clinical trial, our academic collaborators and investigators at the study sites, and our colleagues and collaborators around the world who are dedicating their time to this crucial endeavor,” added Bourla. “We could not have come this far without the tremendous commitment of everyone involved.”
“The first interim analysis of our global Phase 3 study provides evidence that a vaccine may effectively prevent COVID-19. This is a victory for innovation, science and a global collaborative effort,” said Prof. Ugur Sahin, BioNTech co-founder and CEO. “When we embarked on this journey 10 months ago this is what we aspired to achieve. Especially today, while we are all in the midst of a second wave and many of us in lockdown, we appreciate even more how important this milestone is on our path towards ending this pandemic and for all of us to regain a sense of normality. We will continue to collect further data as the trial continues to enroll for a final analysis planned when a total of 164 confirmed COVID-19 cases have accrued. I would like to thank everyone who has contributed to make this important achievement possible.”
The Phase 3 clinical trial of BNT162b2 began on July 27 and has enrolled 43,538 participants to date, 38,955 of whom have received a second dose of the vaccine candidate as of November 8, 2020. Approximately 42% of global participants and 30% of U.S. participants have racially and ethnically diverse backgrounds. The trial is continuing to enroll and is expected to continue through the final analysis when a total of 164 confirmed COVID-19 cases have accrued. The study also will evaluate the potential for the vaccine candidate to provide protection against COVID-19 in those who have had prior exposure to SARS-CoV-2, as well as vaccine prevention against severe COVID-19 disease. In addition to the primary efficacy endpoints evaluating confirmed COVID-19 cases accruing from 7 days after the second dose, the final analysis now will include, with the approval of the FDA, new secondary endpoints evaluating efficacy based on cases accruing 14 days after the second dose as well. The companies believe that the addition of these secondary endpoints will help align data across all COVID-19 vaccine studies and allow for cross-trial learnings and comparisons between these novel vaccine platforms. The companies have posted an updated version of the study protocol at https://www.pfizer.com/science/coronavirus.
Pfizer and BioNTech are continuing to accumulate safety data and currently estimate that a median of two months of safety data following the second (and final) dose of the vaccine candidate – the amount of safety data specified by the FDA in its guidance for potential Emergency Use Authorization – will be available by the third week of November. Additionally, participants will continue to be monitored for long-term protection and safety for an additional two years after their second dose.
No comments:
Post a Comment